Nabota 100U, a scientifically formulated Notulinum Toxin Type A, FDA approved for cosmetic use in reducing facial wrinkles.
Each vial contains 100 units of highly purified neurotoxin complex, ensuring precise and controlled application for effective results. Nabota 100U is specifically designed for durability and stability, offering safe, consistent outcomes for skin rejuvenation.
Product Inquiry Form

Nabota™ From BuyFillers
Nabota 100 is at the leading edge of aesthetic medicine, armed with botulinum toxin type A solution, meticulously designed to eliminate facial wrinkles and fine lines. The neurotoxin has been designed, for medical purposes, with the mechanism of temporarily weakening the muscles of the face, thereby smoothing the skin and giving it a general look against the signs of aging. Each 100-unit vial of Nabota 100 is designed precisely to be pure and potent, offering the best performance. This is ideal for dermatologists and cosmetics professionals; trust the professionals with Nabota 100. The ideal aesthetic solution to keep a face young and pretty, be it crow's feet, lines on the forehead, or glabellar lines.
Why To Choose Nabota™
High Purity
Nabota has a highly purified formulation of botulinum toxin type A. This means there is much less chance that your body may develop resistance to the treatment over some time, therefore giving a steadfast and consistent result.
Rigorous Testing and Approvals
Nabota 100U has undergone extensive clinical trials to establish its safety profile and efficacy. Its production follows stringent manufacturing standards, ensuring consistency and quality in each vial. Nabota has secured rigorous approvals from reputable regulatory bodies worldwide, including:
South Korean Ministry of Food & Drug Safety (MFDS): The initial and primary approval.
US Food and Drug Administration (FDA): A testament to its safety and efficacy for frown lines.
European Medicines Agency (EMA): Approval for use across European Union member states.
These approvals are granted only after comprehensive evaluation of safety data, clinical trial results, and manufacturing processes.
Quick Onset
Nabota may provide visible results within 24-48 hours, with full results often appearing within a week. This is appealing for those who want a fast improvement in their appearance.
Diffusion Control
Nabota is known for its limited diffusion compared to some other botulinum toxins. This offers the benefit of more precise targeting of treatment areas, decreasing the potential for unwanted effects in surrounding muscles.
Nabota™ Specifications
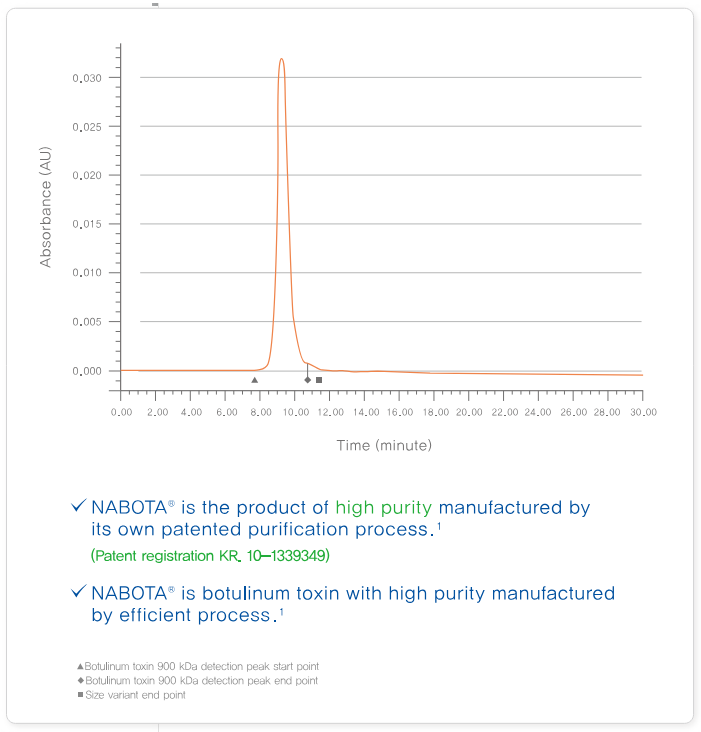
High Purity
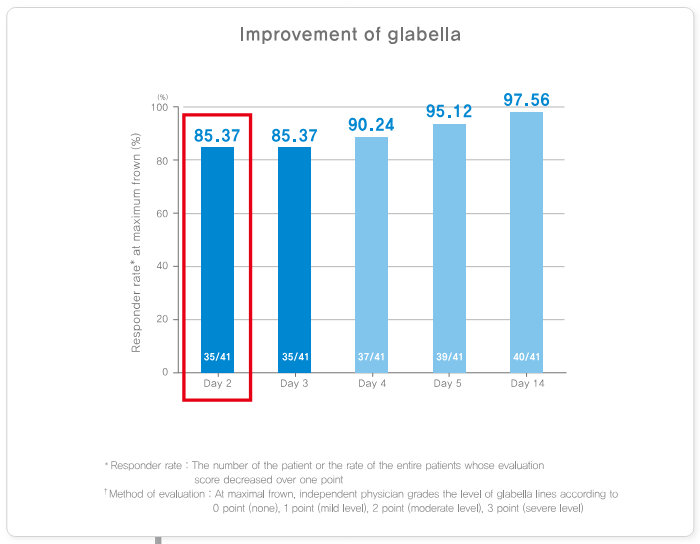
Rapid Improvements
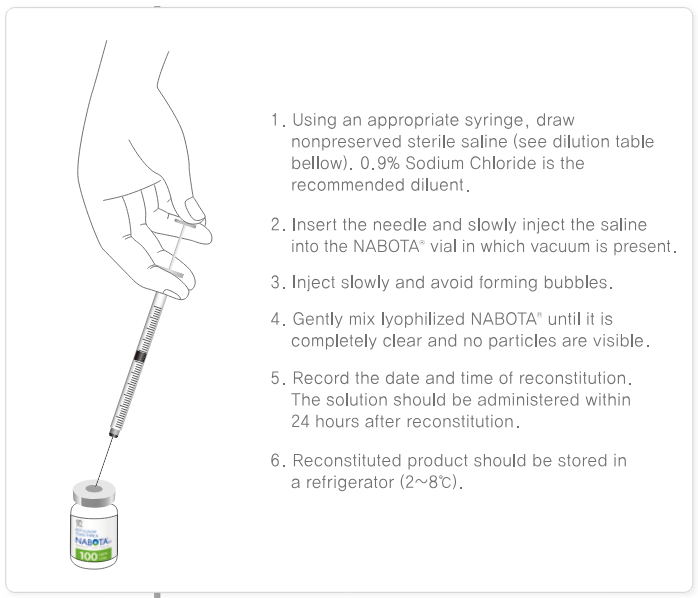
How To Apply Nabota™
Injection sites for Nabota™

FAQ
Yes, Nabota and Botox are both botulinum toxin type A products. They offer similar efficacy and safety profiles, though subtle differences may exist in their effect and duration.
Yes, Nabota 100U, marketed in the U.S. as Jeuveau™, received approval from the U.S. Food and Drug Administration (FDA) in 2019. It is approved for the temporary improvement in the appearance of moderate to severe glabellar lines, commonly referred to as frown lines, in adults.
The effects of Nabota 100 typically last between 3 to 6 months, depending on the individual’s skin type, the area treated, and lifestyle factors. Repeat treatments are recommended to maintain the desired aesthetic effect.
Most people see noticeable improvements within a few days after treatment, with full results becoming apparent within two weeks.
Yes, Nabota 100 can be used in combination with other aesthetic procedures such as fillers, chemical peels, and laser treatments. It’s important to discuss your treatment plan with a qualified professional to ensure safety and effectiveness.
It is primarily approved for frown lines between the eyebrows. It can also be used off-label to treat other wrinkles, such as crow’s feet, forehead lines, bunny lines, and others.
| Ingredient |
Botulinum Toxin Type A |
|---|---|
| Unit | |
| Storage Condition |
2~8℃ |
|
|
Nabota |
|
|